Abstract
Introduction: In the phase 3 MEDALIST trial of luspatercept in lower-risk myelodysplastic syndromes (LR-MDS), 58 of 153 (37.9%) patients who received luspatercept achieved the primary endpoint of red blood cell (RBC) transfusion independence (RBC-TI) ≥ 8 weeks during weeks 1-24, versus 10 of 76 (13.2%) for placebo. Among the primary endpoint responders in the luspatercept arm, 17 (29.3%), 13 (22.4%), and 16 (27.6%) patients experienced 2, 3, or ≥ 4 separate RBC-TI ≥ 8 weeks response events during the entire treatment period, versus 3 (30%) and 1 (10%) patient in the placebo arm, who experienced 2 or 3 response events. (Platzbecker U, et al. Blood 2021;138:1524). The starting dose of luspatercept in the MEDALIST trial was 1.0 mg/kg with up-titration to 1.33 and 1.75 mg/kg allowed in the absence of RBC-TI after ≥ 2 doses at the same dose level, or after a loss of response following a dose reduction.
Here, we report the effect of baseline transfusion burden (TB) and luspatercept dose level on response to treatment in patients with LR-MDS from the MEDALIST study.
Methods: Eligible patients were ≥ 18 years of age, had LR-MDS requiring RBC transfusions, and were ineligible for, intolerant to, or refractory to erythropoiesis-simulating agents. Patients were stratified by mean baseline TB (mean RBC units/8 weeks assessed over the 16 weeks prior to initiation), as follows: low TB (LTB), > 0 to < 4 RBC units/8 weeks; intermediate TB (ITB), ≥ 4 to < 6 RBC units/8 weeks; and high TB (HTB), ≥ 6 RBC units/8 weeks. The number of individual periods of response (distinct episodes of RBC-TI ≥ 8 weeks) occurring during the entire treatment period was calculated. The treatment period lasted until the earliest of: the last dose date + 20 days; study discontinuation; data cutoff; or death. Time between RBC-TI responses was calculated as: start date of next response minus end date of current response plus 1.
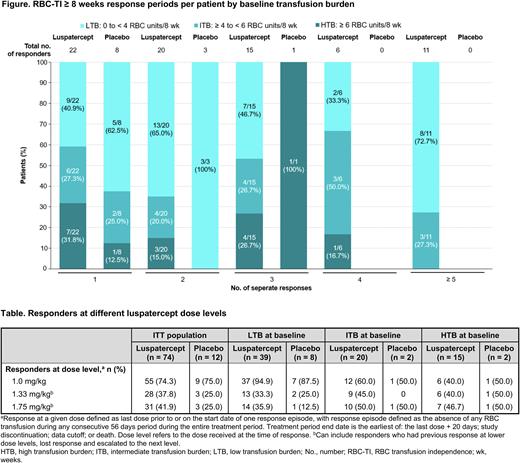
Results: As of November 26, 2020, RBC-TI ≥ 8 weeks was observed in 74/153 (48.4%) luspatercept patients and 12/76 (15.8%) placebo patients during the entire MEDALIST treatment period. Many patients experienced multiple distinct periods of RBC-TI ≥ 8 weeks; 52/74 (70.3%) luspatercept responders and 4/12 (33.3%) placebo responders experienced ≥ 2 episodes. The greatest number of response periods reported in a single patient was 10 with luspatercept and 3 with placebo. Overall, 30/39 (76.9%), 14/20 (70.0%), and 8/15 (53.3%) LTB, ITB, and HTB luspatercept responders, respectively, experienced ≥ 2 periods of response; 17/39 (43.6%), 10/20 (50.0%), and 5/15 (33.3%) LTB, ITB, and HTB luspatercept responders, respectively, experienced ≥ 3 periods of response; and 8/39 (20.5%), 3/20 (15.0%), and 0 LTB, ITB, and HTB, luspatercept responders, respectively, experienced ≥ 5 periods of response (Figure).
The mean (SD) number of response periods with luspatercept was 3.0 (2.25) for LTB patients, 2.9 (2.0) for ITB patients, and 1.9 (1.03) for HTB patients. The greatest number of response periods reported with luspatercept in a single patient was 10 for LTB patients, 8 for ITB patients, and 4 for HTB patients. The mean (SD) time between RBC-TI response periods with luspatercept was 24.3 (63.16) days for LTB patients, 52.1 (55.91) days for ITB patients, and 32.1 (33.79) days for HTB patients.
During the entire treatment period, rates of RBC-TI ≥ 8 weeks response at 1.0 mg/kg decreased with increasing baseline TB, with 94.9% of baseline LTB patients responding compared with 40.0% of baseline HTB patients. Patients who were ITB or HTB at baseline were more likely to respond when receiving 1.75 mg/kg luspatercept, with 50.0% and 46.7% responding, respectively, compared with 35.9% for LTB patients (Table).
Conclusions: Patients who were LTB at baseline experienced more periods of RBC-TI response than ITB and HTB patients. LTB patients were more likely to respond to lower doses of luspatercept, whereas almost half of ITB and HTB patients required escalation to the maximum dose level to respond. ITB and HTB patients might be expected to wait longer for a second response to luspatercept than LTB patients. Importantly, many patients experienced multiple RBC-TI response periods with luspatercept, emphasizing the value of measuring cumulative response duration as well as the benefit of continuing luspatercept treatment.
Disclosures
Platzbecker:Abbvie: Honoraria; BMS/Celgene: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Jazz: Honoraria; Silence Therapeutics: Honoraria; Geron: Honoraria. Santini:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Novartis: Honoraria; Syros: Membership on an entity's Board of Directors or advisory committees; Srvier: Membership on an entity's Board of Directors or advisory committees. Komrokji:CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Acceleron Pharma: Consultancy; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; Servier: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Zeidan:Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding. Garcia-Manero:Genentech: Honoraria, Research Funding; Aprea: Honoraria; Acceleron Pharma: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Curis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; AbbVie: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Buckstein:Takeda: Research Funding; BMS: Honoraria, Research Funding; Taiho: Honoraria, Research Funding. Oliva:BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Patents & Royalties: HM PRO, Speakers Bureau; Janssen: Consultancy; Amgen: Honoraria, Speakers Bureau; Daiichi: Consultancy; Sobi: Honoraria; Apellis: Consultancy, Honoraria. Pozharskaya:BMS: Current Employment. Ha:BMS: Current Employment. Nadal:BMS: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Miteva:BMS: Current Employment. Fenaux:Jazz: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal